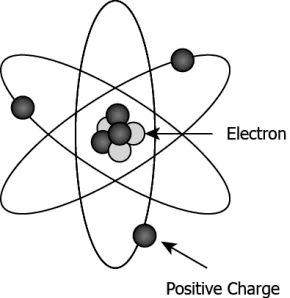
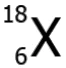Hint: Know the different constituents of an atom and differentiate between electrons and protons.
Question.1. How the sub atomic particles like Proton is different from electron?
(a) An electron is much heavier than a proton.
(b) An electron carries more charge than a proton.
(c) A proton can be easily removed from the atom, but not an electron.
(d) A proton is positively charged, whereas an electron is negatively charged.
Question.2. An atom has 4 electrons and 4 protons and 4 neutrons. The atom is electrically neutral.
Which difference in the properties of protons and electrons affects the electrical neutrality of the atom?
(a) Electrons are 2000 times lighter than protons.
(b) Electrons and protons have the same charge and mass.
(c) Electrons and protons have opposite charges of the same magnitude
(d) Electrons are present in the outer side of the atom compared to the protons.
Ans.1. (d) A proton is positively charged, whereas an electron is negatively charged.
Ans.2. (c) Electrons and protons have opposite charges of the same magnitude
Hint: Explain Thomson’s model of an atom and its incoherent features with the results of other experiments.
Question.3. Which of the following model describes the structure of an atom proposed by Thompson?
(a) 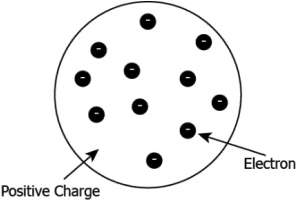
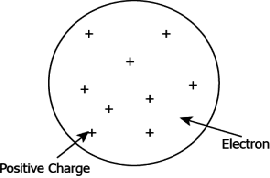
Question.4. Which of the following is NOT the correct explanation of Thompson’s atomic model?
(a) The atom is positively charged sphere.
(b) Electrons are embedded in the positive sphere.
(c) The positive and negative charges balance each other.
(d) Protons and neutrons are embedded in the center of the sphere.
Ans.3. (a) 
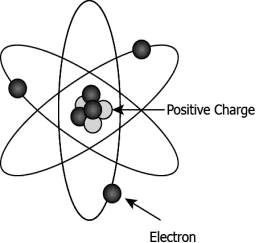
Hint: Draw logical conclusions from Rutherford’s experiment to understand the structure of an atom.
Question.5. Rutherford conducted an experiment to study the structure of atom. He passed positively charged alpha particles through a thin gold foil. He studied the angle of deflection of the alpha particles. He noticed that most of the alpha particles passed through the gold foil without any deflection. What can be concluded from the observation?
(a) Most of the space in an atom is empty.
(b) Atoms have electrons, protons, and neutrons.
(c) The nucleus of the atom is situated at the center.
(d) Atoms do not have positively charged particles.
Question.6. Rutherford observed the following in his experiment.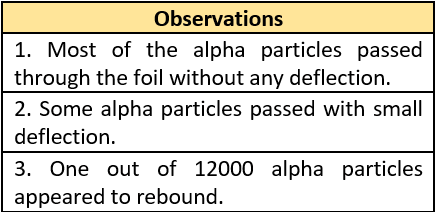
(a) A large space in an atom is mostly empty.
(b) Electrons in an atom revolve around the positively charged protons.
(c) The positive charge and mass of the atom is concentrated at the center of the atom.
(d) Atoms have positively and negatively charged species that are at the center of the atom.
Ans.5. (a) Most of the space in an atom is empty.
Ans.6. (c) The positive charge and mass of the atom is concentrated at the center of the atom.
Hint: Compare Rutherford’s model with Thomson’s atomic model and state their relative advantages and limitations.
Question.7. How Rutherford’s atomic model could explain the drawbacks of Thomson’s atomic model?
(a) It was able to explain that an atom can have multiple electrons and protons.
(b) It was able to explain that an atom has positively and negatively charged particles.
(c) It was able to explain that an atom has a small neutral nucleus and electrons revolve around it.
(d) It was able to explain that an atom has a small positive nucleus and electrons revolve around it.
Question.8. Which of the following is true for Rutherford’s model but not for Thompson’s model of an atom?
(a) An atom is electrically neutral.
(b) An atom contains many electrons.
(c) An atom has positive and negative parts.
(d) An atom contains a positively charged nucleus.
Ans.7. (d) It was able to explain that an atom has a small positive nucleus and electrons revolve around it.
Ans.8. (d) An atom contains a positively charged nucleus.
Hint: Highlight the limitations of Rutherford’s model
Question.9. Which question Rutherford’s model failed to answer?
(a) What causes the atoms to stay electrically neutral?
(b) Where are electrons, protons, and neutrons located in the nucleus?
(c) Why is it easier to remove an electron from an atom compared to a proton?
(d) If electrons revolve continuously, why do they not lose energy and collapse in the nucleus?
Question.10. What fact about electrons was not explained by Rutherford’s model?
(a) Electrons are much lighter than protons.
(b) Electrons are negatively charged species.
(c) Electrons revolve around nucleus but never lose energy.
(d) Electrons can be removed from an atom by providing energy.
Ans.9. (d) If electrons revolve continuously, why do they not lose energy and collapse in the nucleus?
Ans.10. (c) Electrons revolve around nucleus but never lose energy.
Hint: State the postulates of Neils Bohr’s model of an atom and their significance.
Question.11. What part of Bohr’s model of atomic structure explains why electrons do not crash into the nucleus?
(a) Electrons are always repelled by the nucleus.
(b) Electrons are much farther from the nucleus.
(c) Electrons keep gaining energy while revolving.
(d) Electrons revolve in orbits that have specific energy.
Question.12. Which of the following is a postulate of Bohr’s model that explains why electrons do not lose energy as they revolve around the nucleus?
(a) Every atom has a discrete number of orbits in which electrons revolve with fixed energy.
(b) Every atom has a large amount of empty space where the electrons move around nucleus.
(c) Every atom has as many electrons as there are protons which makes it electrically neutral.
(d) Every atom has a positively charged nucleus where most of the mass of the atom is concentrated.
Ans.11. (d) Electrons revolve in orbits that have specific energy.
Ans.12. (a) Every atom has a discrete number of orbits in which electrons revolve with fixed energy.
Hint: Get conversant with the Bohr and Bury rules for distribution of electrons into
different orbits.
Question.13. Which of the following is NOT true about Bohr & Bury’s rule for electronic configuration, where n is the shell no.?
(a) The maximum number of electrons present in a shell is given by the formula 2n^{2}.
(b) The maximum number of electrons present in a shell is given by the formula 2n
(c) The maximum number of electrons that can be accommodated in the outer most orbit is 8.
(d) Electron are not accommodated in a given shell, unless the inner shells are filled
Question.14. Which of the following CAN NOT be the maximum number of electrons in a given shell according to the Bohr & Bury’s rule for electronic configuration:
(a) 2
(b) 6
(c) 18
(d) 32
Ans.13. (b) The maximum number of electrons present in a shell is given by the formula 2n
Ans.14. (b) 6
Hint: Find the valency of elements on the basis of their electronic configuration and
relate inertness and reactivity of elements.
Question.15. The atomic number of sodium (Na) is 11. How many valence electrons does sodium have?
(a) 1
(b) 2
(c) 7
(d) 8
Question.16. The atomic number of Sulphur (S) is 16. Identify the number of electrons in each shell of Sulphur.
(a) 2, 8, 6
(b) 2, 8, 8
(c) 2, 2, 8, 4
(d) 2, 4, 8, 2
Ans.15. (a) 1
Ans.16. (a) 2, 8, 6
Hint: Calculate the number of electrons distributed in different orbits(shells) according to Bohr and Bury rules and find out valence electrons for different elements.
Question.17. The atomic number of some elements is shown.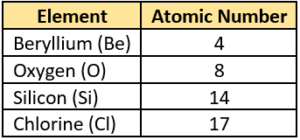
(a) Be and Si
(b) Si and Cl
(c) Be and O
(d) Cl and O
Question.18. Which element would be inert in nature?
(a) Element with 2 electrons
(b) Element with 9 electrons
(c) Element with 12 electrons
(d) Element with 20 electrons
Ans.17. (c) Be and O
Ans.18. (a) Element with 2 electrons
Hint: Write the scientific notations of atoms of commonly known elements and calculate their atomic mass and atomic number.
Question.19. The image shows the scientific notation of sodium.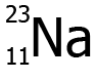
(a) 11
(b) 12
(c) 23
(d) 34
Question.20. An element X has an atomic number 6 and atomic mass 12. Which scientific notation can represent the element?
(a) 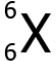
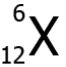
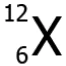
Ans.19. (c) 23
Ans.20. (c)
Hint: Postulate the reason for different atomic numbers for isotopes of an element.
Question.21. Chlorine has two isotopes, Cl-35 and Cl-37. These isotopes occur in nature in the ratio 3:1. What would be the atomic mass for chlorine?
(a) 35.0 u
(b) 35.5 u
(c) 36.0 u
(d) 36.5 u
Question.22. The image shows the isotopes of hydrogen.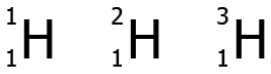
(a) because they have different number of protons
(b) because they have different number of neutrons
(c) because they have different number of electrons
(d) because they have different abundance in nature
Ans.21. (b) 35.5 u
Ans.22. (b) because they have different number of neutrons
Hint: Identify isobars on the basis of scientific notation of their atoms.
Question.23. Look at the given elements.Which of the two are isobars?
(a) Ar and F
(b) Ca and Ar
(c) Ca and Ne
(d) Ne and F
Question.24. Ca and Ar are called isobars because they have the same
(a) mass number but different atomic number.
(b) atomic number but different mass number.
(c) number of protons but different number of neutrons.
(d) number of electrons but different number of protons.
Ans.23. (b) Ca and Ar
Ans.24. (a) mass number but different atomic number.