Hint: Apply the Law of Conservation of Mass to determine the mass of elements in a mixture.
Question.1. A student wants to make a homogeneous mixture of salt, sugar, and water that weighs 300 g. The student has 50 g sugar and 70 g salt. How much water should he add to the mixture?
(a) 70 g
(b) 120 g
(c) 150 g
(d) 180 g
Question.2. A student makes two solutions using components as listed in the table.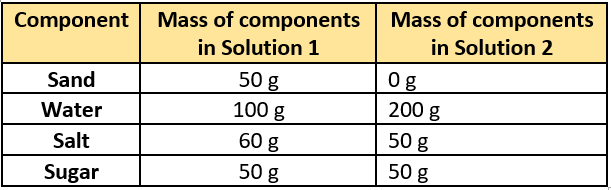
(a) Solution 1, because sand is heavy.
(b) Solution 2, because it has more water.
(c) Solution 1, because it has more salt that disappears in water.
(d) Solution 2, because the total mass of components is greater.
Ans.1. (d) 180 g
Ans.2. (d) Solution 2, because the total mass of components is greater.
Hint: Apply the Law of Constant Proportions to estimate the amount of elements required in a chemical substance and identify postulates of Dalton’s atomic theory.
Question.3. Which option supports Dalton’s atomic theory?
(a) Atoms of an element have identical mass.
(b) Atoms can be created by chemical reactions.
(c) Atoms of different elements have similar chemical properties
(d) Atoms of an element change their properties in chemical reactions.
Question.4. A student has a sample of 200 g of table sugar (sucrose). For 200 g of sucrose, there are 84 g of carbon. Based on Dalton’s atomic theory, how much carbon will be there in 300 g of sucrose?
(Sucrose = C_{12}H_{22}O_{11})
(a) 42 g
(b) 23.8 g
(c) 56 g
(d) 126 g
Ans.3. (a) Atoms of an element have identical mass.
Ans.4. (d) 126 g
Hint: Correlate the fact of invisibility of atoms to the size of atoms.
Question.5. A student argues that atoms are invisible to the eyes. Which option supports the student’s argument?
(a) they exist freely
(b) they are in gaseous form
(c) they are very small in size
(d) they are in constant motion
Question.6. A student finds the atomic radii of some elements.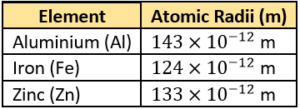
(a) Atoms of only gaseous substances can be observed.
(b) Atoms are very tiny and cannot be observed by a simple hand lens.
(c) Atoms are joined with great force, so it is difficult to distinguish one atom.
(d) Atoms of metals are in constant motion so they cannot be observed with a hand lens.
Ans.5. (c) they are very small in size
Ans.6. (b) Atoms are very tiny and cannot be observed by a simple hand lens.
Hint: List atomic symbols of commonly known elements as per IUPAC.
Question.7. Which symbol is a correct representation of cobalt according to IUPAC?
(a) CO
(b) Co
(c) co
(d) cO
Question.8. A student lists four compounds.
PCl_{5}, KBr, P_{4}O_{10}, Na_{2}CO_{3}
Which compound contains potassium?
(a) KBr
(b) PCl_{5}
(c) P_{4}O_{10}
(d) Na_{2}CO_{3}
Ans.7. (b) Co
Ans.8. (a) KBr
Hint: Recognize that different elements have different atomic mass.
Question.9. The table lists the mass of some atoms.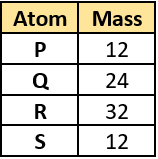
(a) P and Q
(b) R and S
(c) Q and R
(d) P and S
Question.10. The table lists atomic masses and atomic numbers of three elements.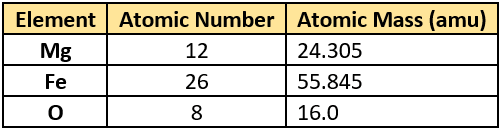
(a) Difference in the atomic mass of iron and magnesium
(b) Difference in the number of iron and magnesium atoms
(c) Difference in the atomic number of iron and magnesium
(d) Difference in the number of O atoms in the two compounds
Ans.9. (d) P and S
Ans.10. (a) Difference in the atomic mass of iron and magnesium
Hint: Determine the number of atoms present in an element on the basis of their atomicity.
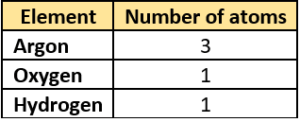
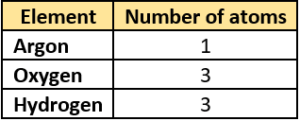
Question.11. The student lists some elements and their atomicity.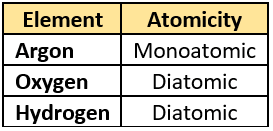
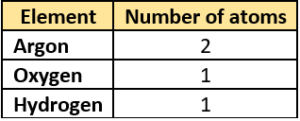
(a) 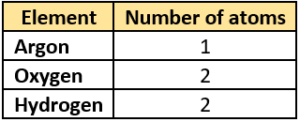
Question.12. The table shows the atomicity of different elements.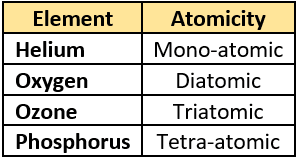
(a) because ozone can exist freely in nature
(b) because ozone is a gas and gases are triatomic
(c) because three atoms of oxygen combine to form ozone
(d) because three molecules of oxygen combine to form ozone
Ans.11. (a) 
Hint: Apply the law of constant proportions to calculate the mass ratio of atoms in a molecule.
Question.13. Which is the correct formula to calculate the mass ratio of ammonia (NH_{3})?
(a) \frac{mass of nitrogen}{(mass of hydrogen)^{3}}
(b) \frac{(mass of nitrogen)^{3}}{mass of hydrogen}
(c) \frac{mass of nitrogen}{3\times mass of hydrogen}
(d) \frac{3\times mass of nitrogen}{mass of hydrogen}
Question.14. The table lists some compounds and their mass ratio.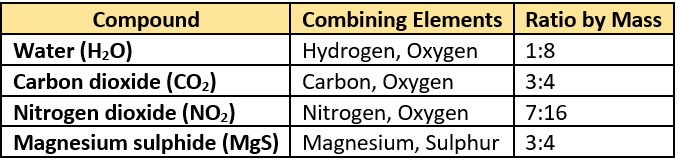
(a) H_{2}O
(b) CO_{2}
(c) NO_{2}
(d) MgS
Ans.13. (c) \frac{mass of nitrogen}{3\times mass of hydrogen}
Ans.14. (b) CO_{2}
Hint: Write chemical formulae using symbols & valencies.
Question.15. A student learns that aluminum forms compound with chlorine and oxygen. She records the valencies of the three elements.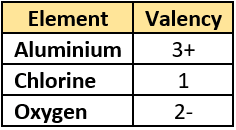
(a) aluminium oxide: Al_{2}O_{3}; aluminium chloride: AlCl_{3}
(b) aluminium oxide: 3AlO_{2}; aluminium chloride: 3AlCl_{2}
(c) aluminium oxide: Al_{3}O_{2}; aluminium chloride: Al_{3}Cl_{2}
(d) aluminium oxide: 3(AlO)_{2}</span>; aluminium chloride: 2(Al_{3}Cl)
Question.16. The table lists valencies of different elements.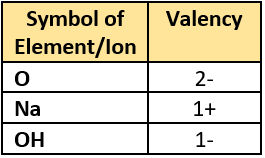
(a) Na_{2}O
(b) NaO_{2}
(c) 2NaOH
(d) 2Na(OH)
Ans.15. (a) aluminium oxide: Al_{2}O_{3}; aluminium chloride: AlCl_{3}
Ans.16. (a) Na_{2}O
Hint: Identify ionic compounds whose formula unit mass can be calculated.
Question.17. The table lists some compounds.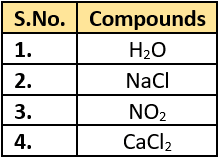
(a) 1 and 3
(b) 2 and 3
(c) 2 and 4
(d) 1 and 2
Question.18. What is the formula unit mass of Na_{2}O?
(a) 20 u
(b) 39 u
(c) 55 u
(d) 62 u
Ans.17. (c) 2 and 4
Ans.18. (d) 62 u
Hint: Calculate the relative molecular mass of commonly known chemical compounds.
Question.19. Which option correctly represents the molecular mass of C_{6}H_{12}O_{6}?
(a) 29 u
(b) 43 u
(c) 96 u
(d) 180 u
Question.20. A student is studying Haber’s process to produce Ammonia. She learns that nitrogen and hydrogen react to form ammonia in the chemical reaction as shown.
N_{2}+3H_{2}\rightleftharpoons 2NH_{3}
Which option gives the ratio of nitrogen and hydrogen that participate in the reaction?
(a) 14:3
(b) 14:1
(c) 28:3
(d) 14:6
Ans.19. (d) 180 u
Ans.20. (a) 14:3
Hint: Calculate the number of moles, mass, atoms and molecules using Avogadro’s number.
Question.21. What will be the number of particles in 36 g of carbon atoms?
(a) 1.003 \times 10^{3}
(b) 2.008 \times 10^{3}
(c) 18.066 \times 10^{3}
(d) 36.132 \times 10^{3}
Question.22. The image shows four samples of elements.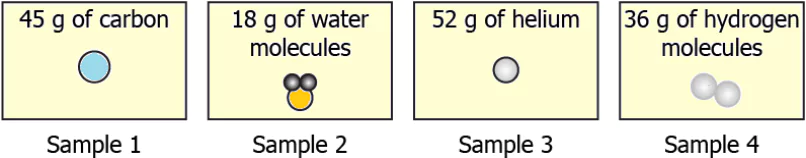
(a) sample 1
(b) sample 2
(c) sample 3
(d) sample 4
Ans.21. (c) 18.066 \times 10^{3}
Ans.22. (d) sample 4