Hint: Identify mixtures from your surroundings based on their characteristic properties.
Question.1. Which of the following can be classified as a mixture?
(a) a clear white salt solution
(b) a rusted iron nail
(c) a piece of paper cut into different shapes
(d) a bowl of water with floating ice cubes
Question.2. During an activity, a student added 10 g iron filings and 10 g sulphur powder in a bowl. He brought a magnet over the bowl and noticed that iron filings were picked up by the magnet.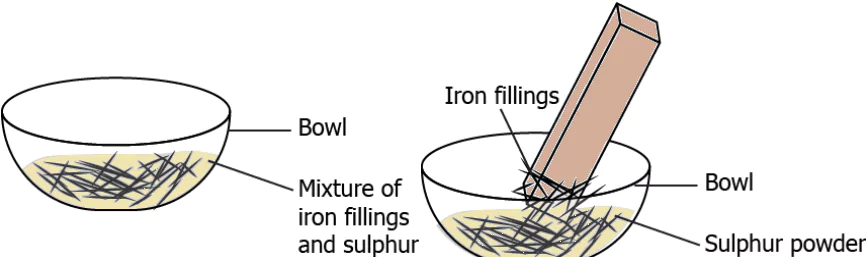
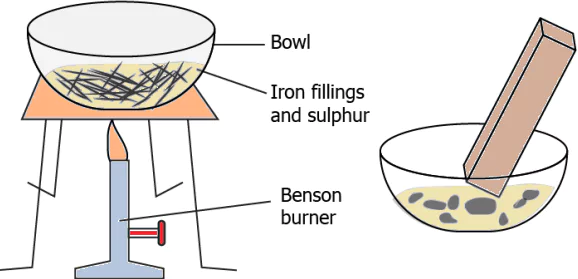
(a) The contents in the bowl before heating can be classified as a mixture because they appear different.
(b) The contents in the bowl before heating can be classified as a mixture because they could be separated.
(c) The contents in the bowl after heating can be classified as a mixture because they were not affected by the magnet.
(d) The contents in the bowl after heating can be classified as a mixture because their properties changed after heating.
Ans.1. (a) a clear white salt solution
Ans.2. (b) The contents in the bowl before heating can be classified as a mixture because they could be separated.
Hint: Differentiate between homogeneous and heterogeneous mixtures.
Question.3. A student is asked to make a homogeneous mixture. He is provided with the following substances.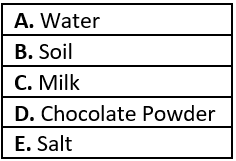
(a) A and B
(b) C and D
(c) B and E
(d) A and E
Question.4. The image shows two solutions.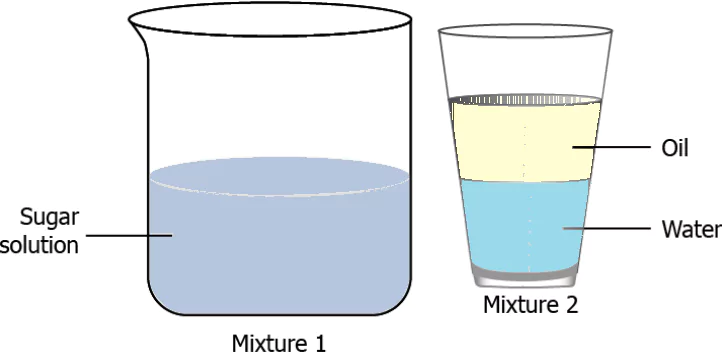
(a) Mixture 2 is homogeneous because the components of a homogenous mixture always form separate layers.
(b) Mixture 1 is homogeneous because the composition is uniform throughout the mixture.
(c) Mixture 2 is heterogeneous because the components of a heterogeneous mixtures are always liquid.
(d) Mixture 1 is heterogeneous because the components of the mixture are not visible from the naked eye.
Ans.3. (d) A and E
Ans.4. (b) Mixture 1 is homogeneous because the composition is uniform throughout the mixture.
Hint: Classify homogenous and heterogenous mixtures into solutions, suspensions and colloids.
Question.5. A student listed some mixtures and classified them into various types.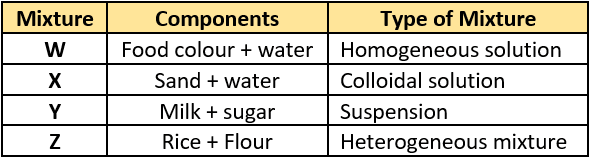
(a) W and X
(b) X and Y
(c) Y and Z
(d) W and Z
Question.6. A student crushed a piece of chalk and mixed the chalk powder in 100 mL water. The water appeared white and cloudy. After some time the particles settled at the bottom of the container. She claims that the mixture is a suspension. What justifies her claim?
(a) The particles mix completely with water.
(b) The particles of chalk form a separate layer.
(c) The particles of chalk are visible through the naked eye.
(d) The particles of chalk are uniformly distributed in water.
Ans.5. (d) W and Z
Ans.6. (c) The particles of chalk are visible through the naked eye.
Hint: Determine the effect of concentration of solution on its physical properties.
Question.7. A student filled two glasses with 100 mL water in each. To glass 1, she added 5 mL orange food colour, and to glass 2 she added 10 mL orange food colour. Which water would appear darker of the two?
(a) Glass 1 because it has less solute
(b) Glass 2 because it has more solute
(c) Glass 1 because it has more solvent
(d) Glass 2 because it has more solvent
Question.8. A student made four solutions using different quantities of water and blue ink. The quantities are listed in the table.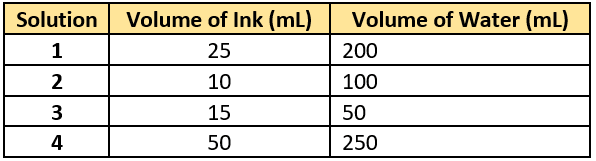
(a) solution 1
(b) solution 2
(c) solution 3
(d) solution 4
Ans.7. (b) Glass 2 because it has more solute
Ans.8. (b) solution 2
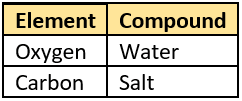
Hint: Classify substances into elements and compounds.
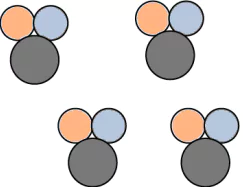
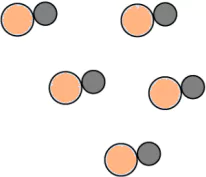
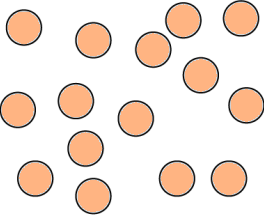
Question.9. A student made models using gumballs. Which model would represent an element?
(a) 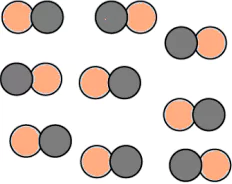
Question.10. The table lists properties of substance X.
(a) Element, because it has different properties.
(b) Element, because it can be divided into smaller parts.
(c) Compound, because it can change from one form to another.
(d) Compound, because it is made of two kinds of atoms joined in definite proportion.
Ans.9. (c)
Ans.10. (d) Compound, because it is made of two kinds of atoms joined in definite proportion.
Hint: Identify the processes to separate various mixtures.
Question.11. A box contains iron nails in saw dust. Which method should be used to separate the two substances?
(a) use a magnet
(b) use a sieve with small holes
(c) use the method of filtration
(d) use hands to pick iron nails out
Question.12. Which separation technique can be used to separate salt from camphor?
(a) sublimation
(b) filtration
(c) magnetic separation
(d) centrifugation
Ans.11. (a) use a magnet
Ans.12. (a) sublimation
Hint: Become conversant with the basis for separation and suggest procedures to separate mixtures of solids in real life situations.
Question.13. Mixture made of two solids can be separated by using various techniques. One of the techniques is adding a solvent, usually water, to the mixture. In which case do we add water to the mixture in order to separate the two solids?
(a) when both solids in the mixture are soluble in water
(b) when both solids in the mixture are insoluble in water
(c) when only one of the components in the mixture is a liquid.
(d) when only one of the solids in the mixture is soluble in water
Question.14. A student dropped iron filings in a bowl of sand. What should the student do to separate the two substances?
(a) Add the mixture in water and then filter the iron filings out.
(b) Mix the mixture with water and put it on heat to evaporate water.
(c) Pass the mixture through a funnel to separate smaller sand particles.
(d) Spread the mixture and bring a magnet over the contents to pick iron filings from the sand.
Ans.13. (d) when only one of the solids in the mixture is soluble in water
Ans.14. (d) Spread the mixture and bring a magnet over the contents to pick iron filings from the sand.
Hint: Identify different processes to separate mixtures and apply them to separate
various mixtures.
Question.15. A student took a solution of salty water. He wants to separate the salt from the water.
Which process would separate the salt from the water?
(a) filtration
(b) evaporation
(c) centrifugation
(d) chromatography
Question.16. Which mixture correctly aligns with their method of separation?
(a) oil and water filtration
(b) cream from milk distillation
(c) salt and water evaporation
(d) salt and sand centrifugation
Ans.15. (b) evaporation
Ans.16. (c) salt and water evaporation
Hint: Suggest procedures to separate mixtures of solids and liquids.
Question.17. A student studies that cream is obtained from milk. He also understands that cream is solid, and milk is liquid. Moreover, particles of cream are very small allowing them to pass through a filter paper. Which process should he adopt to separate the cream from the milk?
(a) filtration, as milk is liquid and hence it can easily be filtered out
(b) filtration, as the cream is solid and hence it can easily be filtered out
(c) centrifugation, as cream and milk have a difference in their chemical formula
(d) centrifugation, as particles of cream and milk have a difference in their density
Question.18. The image represents a mixture of sand and water.
(a) filtration
(b) centrifugation
(c) crystallization
(d) chromatography
Question.19. Which option explains the process of filtration?
(a) it allows only liquid to pass from a mixture
(b) it allows only solid particles to pass from a mixture
(c) it allows the separation of substances based on colour
(d) it allows the separation of substances based on density
Question.20. A student collected a mixture of sawdust and water to conduct an experiment. In order to separate the sawdust from the mixture, he poured the water through a funnel where a filter paper was already placed. He observed that the solid is collected in the funnel whereas the liquid is collected in the beaker below. What causes the separation of the mixture?
(a) absorption of water into the filter paper
(b) absorption of sawdust into the filter paper
(c) allowing liquid to pass through the filter paper
(d) allowing sawdust to pass through the filter paper
Ans.17. (d) centrifugation, as particles of cream and milk have a difference in their density
Ans.18. (a) filtration
Ans.19. (a) it allows only liquid to pass from a mixture
Ans.20. (c) allowing liquid to pass through the filter paper
Hint: State the underlying principle behind centrifugation process and locate its use.
Question.21. The table lists a few properties of substances:
(a) only colour
(b) only density
(c) both luster and colour
(d) both colour and density
Question.22. A student studies that by using centrifugation process cream can be separated from milk. He also understands that cream is solid, whereas milk is a liquid. Both of these particles are very small which allows them to pass through a filter paper but vary in their densities. Why is the centrifugation process helpful in separating such mixture?
(a) as the substances vary in colour
(b) as the substances have a difference in density
(c) as the substances are completely soluble in nature
(d) as the substance have a difference in boiling temperature
Ans.21. (b) only density
Ans.22. (b) as the substances have a difference in density
Hint: Identify situations where basic principle of evaporation is used to separate solids from liquids.
Question.23. Aayush puts his wet clothes under the sun and observes that his clothes get warm and dries after some time. Which process helped him in this process?
(a) evaporation as water changes from liquid to solid
(b) evaporation as water changes from liquid to vapor
(c) crystallization as water changes from liquid to solid
(d) crystallization as water changes from liquid to vapor
Question.24. Hassan wants to separate sodium chloride dissolved in water. He uses evaporation for the separation. How does the process of evaporation help Hassan?
(a) Sodium chloride will change from solid to vapor and can be extracted.
(b) Sodium chloride will change from liquid to vapor and can be separated.
(c) Water will change from vapor to liquid and sodium chloride can be extracted.
(d) Water will change from liquid to vapor and sodium chloride can be extracted.
Ans.23. (b) evaporation as water changes from liquid to vapor
Ans.24. (d) Water will change from liquid to vapor and sodium chloride can be extracted.
Hint: Explain the process of crystallization.
Question.25. Which option describes the process of crystallization?
(a) It involves the formation of crystals after solute evaporates.
(b) It involves the formation of crystals after the solvent evaporates.
(c) It involves the decomposition of crystals after solute evaporates.
(d) It involves the decomposition of crystals after the solvent evaporates.
Question.26. A student performs an experiment in which the copper sulphate solution was heated in a china dish.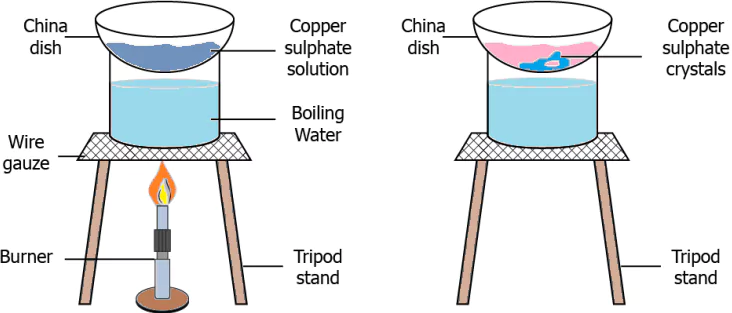
(a) filtration as it results in the formation of precipitate on heating
(b) evaporation as heating evaporates the solute from the solution
(c) crystallization as solid copper sulphate is left in the china dish
(d) distillation as the colour of copper sulphate changed on heating
Ans.25. (b) It involves the formation of crystals after the solvent evaporates.
Ans.26. (c) crystallization as solid copper sulphate is left in the china dish
Hint: Deduce the process of chromatography and identify mixtures that can be separated by the use of this procedure.
Question.27. Daisy wants to separate the coloured components of a dye as a part of a lab experiment.
The dye contains water and coloured components. Which process should she perform?
(a) Evaporation as water changes from liquid to vapor.
(b) Crystallization as water changes from vapour to solid.
(c) Distillation as it separates substance based on boiling point.
(d) Chromatography as it separates the solutes that dissolve in the same solvent.
Question.28. Himanshu wants to separate two photosynthetic pigments P and Q from the plant extract. He uses paper chromatography for the separation of these pigments and observes pigment P is separated first. What can be a likely reason for the same?
(a) Pigment P has the low density.
(b) Pigment Q has the high density.
(c) Pigment P is more soluble in water and rises faster.
(d) Pigment Q is more soluble in water and rises slower.
Ans.27. (d) Chromatography as it separates the solutes that dissolve in the same solvent.
Ans.28. (c) Pigment P is more soluble in water and rises faster.
Hint: Recognize the basis of distillation and identify mixtures that can be separated
through this.
Question.29. Which method can be used to separate two liquids (different boiling point) from their mixture?
(a) Distillation, as it separates liquid based on boiling point.
(b) Evaporation, as it separates liquid based on melting point.
(c) Chromatography as it separates components that have low melting point.
(d) Centrifugation as it separates components that have higher boiling point.
Question.30. A student wants to separate acetone and water from their mixture. The boiling temperature of acetone is higher than the boiling temperature of water. Which technique should she perform?
(a) Distillation, as it separates liquids based on their boiling point.
(b) Evaporation, as it separates liquids based on their boiling point.
(c) Distillation, as it separates liquid based on their melting point.
(d) Evaporation, as It separates liquids based on their melting point.
Ans.29. (a) Distillation, as it separates liquid based on boiling point.
Ans.30. (a) Distillation, as it separates liquids based on their boiling point.
Hint: Identify suitable processes of separation to separate mixtures in real life
situations.
Question.31. A student has a mixture of sand, water, and salt. What is the order of separation techniques that he must perform to collect salt?
(a) Filtration → Evaporation → Crystallisation
(b) Filtration → Centrifugation → Crystallisation
(c) Evaporation → Filtration → Chromatography
(d) Chromatography → Centrifugation → Crystallisation
Question.32. A student performed an experiment to analyse the salt present in a sample. He passed the mixture through a sieve and collects the residue. The residue was kept on a porous funnel and the funnel was placed in a machine. The machine rotated the funnel and the liquid from the residue passed through the funnel while the solid salt was left on it. The solid was then kept under various instruments to check for the salt. Which two separation techniques did the student perform?
(a) filtration followed by distillation
(b) evaporation followed by filtration
(c) centrifugation followed by filtration
(d) filtration followed by centrifugation
Ans.31. (a) Filtration → Evaporation → Crystallisation
Ans.32. (d) filtration followed by centrifugation
Hint: Explain the principle of chromatography and identify mixtures that can be separated through this process.
Question.33. A student wants to separate the components of a dye. He studies that a specific technique is used to perform the experiment. Which option explains the technique that the student must perform?
(a) Evaporation as the denser components will evaporate leaving lighter components.
(b) Distillation as the different colours will separate when heated at specific
temperatures.
(c) Filtration as the immiscible components will separate out easily when the solution is passed through a filter paper.
(d) Chromatography as the coloured component that is more soluble in water will rise faster and, in this way, the components will get separated.
Question.34. For which mixtures the technique of chromatography can be employed?
(a) It is used for the separation of those solutes that dissolve in the same solvent.
(b) It is used for the separation of those solutes that are solid at room temperature.
(c) It is used for the separation of the components of the mixture that have a similar density.
(d) It is used for the separation of the components of the mixture that have a high melting point.
Ans.33. (d) Chromatography as the coloured component that is more soluble in water will rise faster and, in this way, the components will get separated.
Ans.34. (a) It is used for the separation of those solutes that dissolve in the same solvent.
Hint: Elaborate the technique of fractional distillation to separate homogeneous (or
miscible) liquids.
Question.35. A researcher uses fractional distillation to separate the components of crude oil. Why she chose this method?
(a) crude oil has components that have very less difference in their mass
(b) crude oil has components that have very less difference in their density
(c) crude oil has components that have very less difference in their boiling points
(d) crude oil has components that have very less difference in their melting points
Question.36. How does a fractional column help in the separation of a miscible liquid solution in fractional distillation?
(a) It helps to separate the liquids that have a difference of less than 25K in their boiling points.
(b) It helps to separate the liquids that have a difference of less than 100K in their boiling points.
(c) It helps to separate the liquids that have a difference of more than 100K in their boiling points.
(d) It helps to separate the liquids that have a difference of more than 25K in their boiling points.
Ans.35. (c) crude oil has components that have very less difference in their boiling points
Ans.36. (a) It helps to separate the liquids that have a difference of less than 25K in their boiling points.
Hint: Differentiate between distillation and fractional distillation procedures.
Question.37. How distillation differs from fractional distillation, even though both are evaporation & condensation?
(a) a distillation setup has a heating source
(b) a fractional distillation setup has a water condenser
(c) a distillation setup does not have a fractional column
(d) a fractional distillation setup does not have a distillation flask
Question.38. A student wants to separate two liquids that differ in the boiling point. After learning the boiling point of the two liquids, he chose to perform fractional distillation. What differentiates the two techniques?
(a) fractional distillation is performed on samples that have a boiling point less than 100 K
(b)fractional distillation is performed on samples that have a boiling point higher than 100 K
(c) fractional distillation is performed on samples that have a difference of less than 25 K in boiling point
(d) fractional distillation is performed on samples that have a difference of more than 25 K in boiling point
Ans.37. (c) a distillation setup does not have a fractional column
Ans.38. (c) fractional distillation is performed on samples that have a difference of less than 25 K in boiling point
Hint: Explain the basis for use of separating funnel to separate miscible liquids.
Question.39. The separating funnel is used to separate the components of the liquid solution. Which two components of the solution can be separated by the separating funnel?
(a) oil and water
(b) milk and water
(c) sugar and water
(d) color pigment and water
Question.40. A student uses a separating funnel to separate the mixture of liquid X and Y. How the layers of X and Y separates in the funnel?
(a) the liquids separate based on their densities
(b) the liquids separate based on their temperature
(c) the liquids separate based on their boiling point
(d) the liquids separate based on their melting point
Ans.39. (a) oil and water
Ans.40. (a) the liquids separate based on their densities
Hint: Describe the process of using fractional distillation to explain its use in separating gases from the air.
Question.41. A student wants to separate gases from air. The student asked his teacher, the teacher suggested to use fractional distillation to separate gases from air. How the fractional distillation is suitable to separate gases from air?
(a) it arranges the gases in the column depending on their density
(b) it arranges the gases in the column depending on their boiling point
(c) it arranges the gases in the column depending on their freezing point
(d) it arranges the gases in the column depending on their temperature
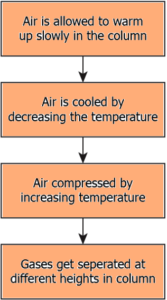
Question.42. Which option explains the process of fractional distillation to separate the gases from air?
(a) 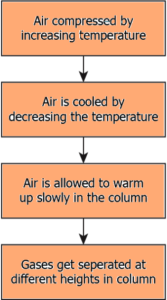
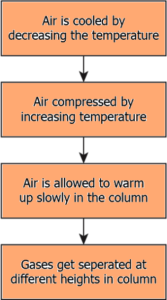
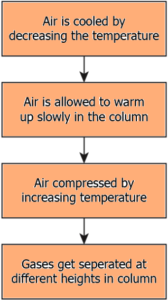
Ans.41. (b) it arranges the gases in the column depending on their boiling point
Ans.42. (a)
Hint: Classify different substances based on their physical properties as metals, non-metals and metalloids.
Question.43. A person has two objects X and Y. The student hit the objects X and Y. The object X makes the ringing sound, but Y does not make the ringing sound. What are X and Y?
(a) X: iron; Y: coal
(b) X: iron; Y: coke
(c) X: coal; Y: coke
(d) X: iron; Y: copper
Question.44. A student listed some items in a table as shown.
(a) 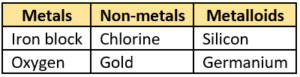
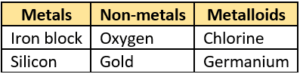

Ans.43. (a) X: iron; Y: coal
Ans.44. (c)
Hint: Differentiate between elements and compounds to classify different materials based on their physical and chemical properties.
Question.45. A student listed some items as shown.
(a) methane and silicon
(b) silicon and hydrogen
(c) acetic acid and hydrogen
(d) methane and acetic acid
Question.46. The table shows the materials.
compounds?
(a) 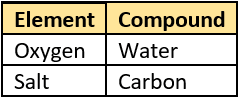
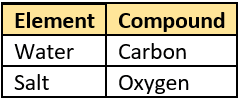
Ans.45. (b) silicon and hydrogen
Ans.46. (b)