Assertion Reason Questions
For question given below, two statements are given- one labelled Assertion (A) and the other labelled Reason (R). Select the correct answer to these questions from the codes (a), (b), (c) and (d) as given below:
(a) Both A and R are true and R is correct explanation of the assertion.
(b) Both A and R are true but R is not the correct explanation of the assertion.
(c) A is true but R is false.
(d) A is false but R is true.
Question.1.
Assertion: Calcium sulphate hemihydrate, CaSO_{4}\cdot \frac{1}{2}H_{2}O is called plaster of Paris.
Reason: Plaster of Paris is used for producing moulds for pottery and ceramics and casts of statues.
Ans.1. (b)
Question.2.
Assertion: Phosphoric acid is a weak acid.
Reason: Phosphoric acid when dissolved in water dissociates partially and produces very little H^{+} ions.
Ans.2. (a)
Question.3.
Assertion: Antacids neutralize the effect of extra acid produced in the stomach during indigestion and thus provide relief.
Reason: Antacids are mild bases.
Ans.3. (a)
Question.4.
Assertion: HCl is a stronger acid than acetic acid.
Reason: On dissociation, HCl yields lesser hydrogen ions for the same concentration as compared to acetic acid.
Ans.4. (c)
On dissociation, HCl yields more hydrogen ions for the same concentration as compared to acetic acid.
Question.5.
Assertion: pH = 7 signifies pure water.
Reason: pH of acetic acid is greater than 7.
Ans.5. (c)
pH of acetic acid is less than 7.
Question.6.
Assertion: pH of ammonium nitrate solution is acidic.
Reason: Solution of a salt of weak base and strong acid is acidic.
Ans.6. (a)
Ammonium nitrate is a salt of ammonium hydroxide (weak base) and nitric acid (strong acid).
Question.7.
Assertion: Acetic acid does not act as an acid in benzene solution.
Reason: Benzene is non-polar.
Ans.7. (a)
For ionization of an acid or base, polar solvents (like water) are required. As ionization does not take place in non-polar solvents (like benzene) so acetic acid does not act as an acid.
Question.8.
Assertion: Bleaching powder reacts with dilute acids to evolve chlorine.
Reason: The chlorine liberated by the action of dilute acids on bleaching powder is called available chlorine.
Ans.8. (b)
The reaction involved is,
CaOCl_{2}+H_{2}SO_{4(dilute)} \rightarrow CaSO_{4}+H_{2}O+Cl_{2}(\uparrow)
Question.9.
Assertion: Sodium carbonate pentahydrate is also known as washing soda.
Reason: Chief raw materials for the manufacture of washing soda are NH_{3} , NaCl and CaCO_{3} .
Ans.9. (d)
Washing soda is sodium carbonate decahydrate, Na_{2}CO_{3}\cdot 10H_{2}O .
Question.10.
Assertion: Common salt is used for the preparation of many chemicals such as sodium hydroxide, bleaching powder, baking soda, washing soda etc.
Reason: Main source of sodium chloride is sea water.
Ans.10. (b)
Question.11.
Assertion: AlCl_{3} is a basic salt.
Reason: AlCl_{3} is a salt of strong acid and a weak base.
Ans.11. (d)
AlCl_{3} is an acidic salt as it is a salt of strong acid ( HCl ) and a weak base Al(OH)_{3} .
Question.12.
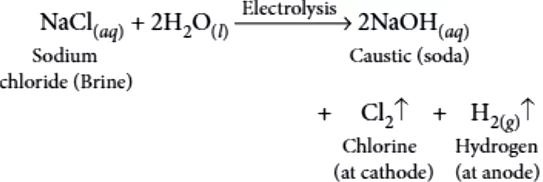
Assertion: Baking soda is prepared by chlor-alkali process.
Reason: Brine decomposes to sodium hydroxide on passing electricity through it.
Ans.12. (d)
Caustic soda (sodium hydroxide, NaOH ) is prepared by chlor-alkali process. Brine decomposes to sodium hydroxide. Chlorine gas is formed at the anode and hydrogen gas at the cathode. Sodium hydroxide solution is formed near the cathode.
Question.13.
Assertion: Salt of KMnO_{3} is formed by strong base and weak acid.
Reason: Salt of NH_{4}Cl is formed by weak base and strong acid.
Ans.13. (d)
Strong Base + Strong acid → salt + water
Eg. KOH+HNO_{3} \rightarrow KNO_{3}+H_{2}O
Weak Base + Strong acid → salt + water
Eg. NH_{4}OH+HCl \rightarrow NH_{4}Cl+H_{2}O
Question.14.
Assertion: Strength of the acid or base decreases with dilution.
Reason: Ionization of an acid or a base increases with dilution.
Ans.14. (b)
Ionization of an acid or a basic increases on dilution but concentration of H^{+} or OH^{-} ions decreases per unit volume, thus strength of the acid or the base decreases with dilution.
Question.15.
Assertion: Higher the H^{+} ion concentration, lower is the pH value.
Reason: The pH of a neutral solution = 7, that of a basic solution < 7 and that of an acidic solution > 7.
Ans.15. (c)
Higher the H^{+} ion concentration, lower is the pH value. The pH value less than 7 represents an acidic solution and value more than 7 represents a basic solution.
Question.16.
Assertion: CH_{3}COOH is used as vinegar in cooking and food preservatives.
Reason: Strong acids are those acids which ionise almost completely in aqueous solution and hence produce a large amount of H^{+} ions.
Ans.16. (b)
Question.17.
Assertion: Tooth decay starts when the pH of the mouth is lower than 5.5.
Reason: Enamel starts corroding below 5.5 pH.
Ans.17. (a)
Question.18.
Assertion: The chemical name of bleaching powder is calcium oxychloride.
Reason: Bleaching powder is used as an oxidising agent in chemical industries.
Ans.18. (b)
Question.19.
Assertion: The process of dissolving an acid or a base in water is highly exothermic reaction.
Reason: Water must always be added slowly to acid with constant stirring.
Ans.19. (c)
The process of dissolving an acid or a base in water is highly exothermic reaction. Acid must always be added slowly to water with constant stirring.
Question.20.
Assertion: Phenolphthalein is an acid-base indicator.
Reason: Phenolphthalein gives different colours in acidic and basic medium.
Ans.20. (a)
Phenolphthalein is a weak organic acid and may be represented as HPh.
HPh_{(Colourless)} \rightleftharpoons H_{(Colourless)}^{+} + Ph_{(Pink)}^-
In acidic medium, excess of H^{+} ions are present and so equilibrium is towards left and hence solution is colourless. While in basic medium, OH^{-} ions combine with H^{+} ions to form unionised water molecules and so equilibrium is towards right and hence solution has pink colour. Therefore, phenolphthalein is an acid-base indicator.