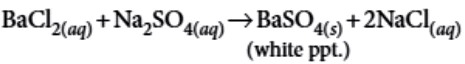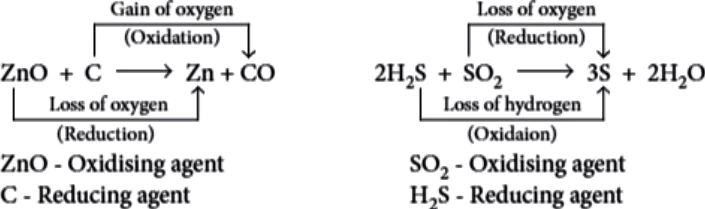Case Study Question 01
Read the following and answer any four questions from 1 to 5.
In a chemical reaction, reactants are converted into products. The conversion of reactants into products in a chemical reaction is often accompanied by some features which can be observed easily. These easily observed features which take place as a result of chemical reaction are known as characteristics of chemicals reactions. Some important characteristics of chemical reactions are:
(I) Evolution of heat
(II) Formation of precipitate
(III) Change in colour
(IV) Change in temperature
(V) Change in state
Any one of these general characteristics can tell us whether a chemical reaction has taken place or not.
Question.1. Reaction of magnesium with air is a/an
(a) exothermic reaction
(b) endothermic reaction
(c) reversible reaction
(d) substitution reaction.
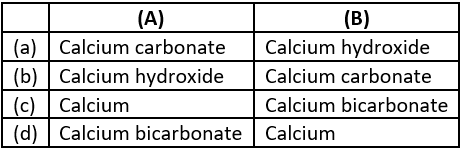
Question.2. In the following reaction,
Ca_{aq}^{2+}+2OH_{aq}^- \rightarrow Ca(OH)_{2(s)}
precipitate of calcium hydroxide will be of
(a) green colour
(b) blue colour
(c) brown colour
(d) white colour
Question.3. In the given reaction,
S_{(s)}+O_{2(g)} \rightarrow SO_{2}
the physical state of SO_{2} is
(a) liquid
(b) solid
(c) gaseous
(d) all three.
Question.4. Which one of the following processes involve chemical reactions?
(a) Storing of oxygen gas under pressure in a gas cylinder.
(b) Keeping petrol in a china dish in the open.
(c) Liquefaction of air.
(d) Heating copper wire in the presence of air at high temperature.
Question.5. In which of the following reactions, high amount of heat energy will be evolved?
(a) Electrolysis of water
(c) Burning of L.P.G.
(b) Dissolution of NH_{4}Cl in water
(d) Decomposition of AgBr in the presence of light
Ans.1. (a)
Ans.2. (d)
Calcium hydroxide is a white colour solid.
Ans.3. (c)
SO_{2} is gaseous in nature.
Ans.4. (d)
When copper is heated in the presence of air in a very high temperature, a chemical reaction takes place. Copper reacts with oxygen of the air to form a thin layer of copper oxide on the surface of metallic copper.
Ans.5. (c)
On burning of L.P.G., heat is evolved.
Case Study Question 02
Read the following and answer any four questions from 1 to 5.
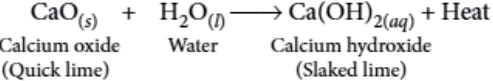
A reaction in which two or more reactants combine to form a single product is called a combination reaction. For example, calcium oxide reacts vigorously with water to form calcium hydroxide. The reaction is highly exothermic in nature, as lots of heat is produced during the reaction.
CaO_{(s)}+H_{2}O_{(l)} \rightarrow Ca(OH)_{2(aq)}+Heat
Solution of Ca(OH)_{2} is used for white wash the walls. Calcium hydroxide reacts slowly with carbon dioxide in air to form a thin layer of calcium carbonate on the wall which gives a shiny appearance to wall. Calcium carbonate will form after two or three days of white wash.
Question.1. What is the chemical name of quick lime?
(a) Calcium oxide
(b) Calcium carbonate
(c) Calcium hydroxide
(d) Carbon dioxide
Question.2. When carbon dioxide is passed through lime water,
(a) calcium hydroxide is formed
(b) white precipitate of CaO is formed
(c) lime water turns milky
(d) colour of lime water becomes green
Question.3. Following observations are observed when calcium oxide reacts vigorously with water.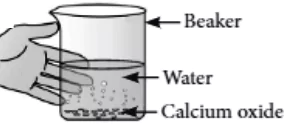
(I) It is an endothermic reaction.
(II) Slaked lime is produced.
(III) Quick lime is produced.
(IV) It is an exothermic reaction.
(V) It is a combination reaction.
(a) (I) and (II)
(b) (III) and (IV)
(c) (I) and (III)
(d) (II), (IV) and (V)
Question.4. Quick lime combines vigorously with water to form (A) which reacts slowly with the carbon dioxide in air to form (B).
Identify the compounds(A) and (B).
Question.5. Among the following, the endothermic reaction is
(a) combination of carbon and oxygen to form carbon monoxide
(b) combination of nitrogen and oxygen to form nitrogen monoxide
(c) combination of glucose and oxygen to form carbon dioxide and water
(d) combination of zinc and hydrochloric acid to form zinc chloride and hydrogen.
Ans.1. (a)
Calcium oxide (CaO) is quick lime.
Ans.2. (c) 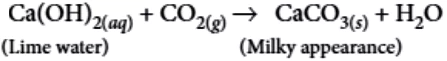
Calcium oxide (quick lime) reacts vigorously with water to produce calcium hydroxide (slaked lime) releasing a large amount of heat. It is a combination reaction.
Ans.4. (b) 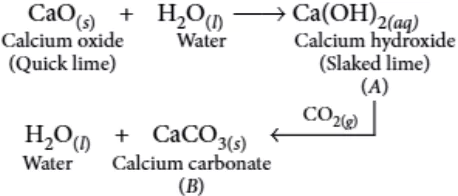
Combination of N_{2} and O_{2} to form NO is an endothermic reaction with absorption of heat.
N_{2(g)}+O_{2(g)}+Heat \rightarrow 2NO_{(g)}
Case Study Question 03
Read the following and answer any four questions from 1 to 5.
Reactions in which one element takes place of another element in a compound, are known as displacement reactions. In general, more reactive elements displaces a less reactive element from its compound. In all single displacement reactions, only one element displaces another element from its compound. The single displacement reactions are, however, written as just displacement reactions. The displacement reaction between iron (III) oxide and powdered aluminium produces so much heat that iron metal obtained is in molten form.
Question.1. Copper displaces which of the following metals from its salt solution?
(a) ZnSO_{4}
(b) FeSO_{4}
(c) AgNO_{3}
(d) NiSO_{4}
Question.2. When zinc reacts with dilute sulphuric acid, the gas evolved is
(a) red in colour and have a sweet smelling.
(b) green in colour and have a foul smell.
(c) colourless, odourless and burns with a pop sound.
(d) colourless, pungent smelling and burns with a pop sound.
Question.3. When dry hydrogen is passed over a heated oxide of metal X using the apparatus shown below, a reddish- brown residue is obtained.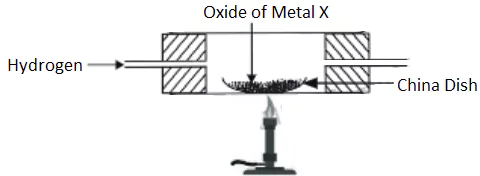
(a) copper
(b) lead
(c) silver
(d) zinc.
Question.4. Which of the following reactions is a displacement reaction?
(a) CaO+H_{2}O \rightarrow Ca(OH)_{2}
(b) MgCO_{3} \rightarrow Mg+CO_{2}
(c) Mg+CuSO_{4} \rightarrow MgSO_{4}+Cu
(d) H_{2}+Cl_{2} \rightarrow 2HCl
Question.5. When dilute hydrochloric acid is added to granulated zinc placed in a test tube, the observation made is
(a) the surface of the metal turns shining
(b) the reaction mixture turns milky
(c) greenish yellow gas is evolved
(d) the colourless and odourless gas evolves with a pop sound.
Ans.1. (c)
Cu+2AgNO_{3} \rightarrow Cu(NO_{3})_{2}+2Ag
Copper can displace silver from its salt solution since, copper is more reactive than silver.
Ans.2. (c)
Zn+H_{2}SO_{4(dil.)} \rightarrow ZnSO_{4}+H_{2}\uparrow
H_{2} is a colourless, odourless gas and burns with a pop sound.
Ans.3. (a) 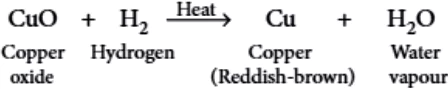
It is a single displacement reaction.
Ans.5. (d)
Zn+2HCl \rightarrow ZnCl_{2}+H_{2}\uparrow
Case Study Question 04
Read the following and answer any four questions from 1 to 5.
Those reactions in which two compounds react by an exchange of ions to form two new compounds are called double displacement reactions. A double displacement reaction usually occurs in solution and one of the products, being insoluble, precipitate out (separates as a solid). Any reaction in which an insoluble solid (called precipitate) is formed that separates from the solution is called a precipitation reaction. The reaction in which acid or acidic oxide reacts with base or basic oxide to form salt and water is called neutralisation reaction. For example,
2NaOH+H_{2}SO_{4} \rightarrow Na_{2}SO_{4}+H_{2}O
Question.1. When hydrogen sulphide gas is passed through a blue solution of copper sulphate, a black precipitate of copper sulphide is obtained and the sulphuric acid so formed remains in the solution. The reaction is an example of a
(a) combination reaction
(b) displacement reaction
(c) decomposition reaction
(d) double displacement reaction.
Question.2. Which of the following is not a double displacement reaction?
(a) AgNO_{3(aq)}+NaCl_{(aq)} \rightarrow AgCl_{(s)}+NaNO_{3(aq)}
(b) Zn_{(s)}+H_{2}SO_{4(aq)} \rightarrow ZnSO_{4(aq)}+H_{2(g)}
(c) CuSO_{4(aq)}+H_{2}S_{(aq)} \rightarrow CuS_{(s)}+H_{2}SO_{4(aq)}
(d) Pb(NO_{3})_{2(aq)}+2KI_{(aq)} \rightarrow PbI_{2(s)}+2KNO_{3(aq)}
Question.3. Barium chloride on reaction with ammonium sulphate forms barium sulphate and ammonium chloride. Which of the following correctly represents the type of the reaction involved?
(I) Displacement reaction
(II) Precipitation reaction
(III) Combination reaction
(IV) Double displacement reaction
(a) (I) only
(b) (II) only
(c) (III) and (IV) only
(d) (II) and (IV) only
Question.4. Identify A in the following reaction.
AlCl_{3(aq)}+3NH_{4}OH_{(aq)} \rightarrow A+3NH_{4}Cl_{(aq)}
(a) Al(OH)_{3}
(b) Al_{2}O_{3}
(c) AlH_{3}
(d) AlN
Question.5. Consider the following reaction,
BaCl_{2}+Na_{2}SO_{4} \rightarrow BaSO_{4}+2NaCl
identify the precipitate in the reaction.
(a) BaCl_{2}
(b) BaSO_{4}
(c) Na_{2}SO_{4}
(d) NaCl
Ans.1. (d)
CuSO_{4}+H_{2}S \rightarrow CuS+H_{2}SO_{4}
Both CuSO_{4} and H_{2}S exchange their ions to give new compounds- CuS and H_{2}SO_{4}. Hence, this is a double displacement reaction.
Ans.2. (b)
It is an example of single displacement reaction.
Ans.3. (d)
BaCl_{2}+(NH_{4})_{2}SO_{4} \rightarrow BaSO_{4}(\downarrow)+2NH_{4}Cl
It is a precipitation reaction as well as double displacement reaction.
Ans.4. (a)
AlCl_{3}+3NH_{4}OH \rightarrow Al(OH)_{3}+3NH_{4}Cl
Ans.5. (b)
Case Study Question 05
Read the following and answer any four questions from 1 to 5.
The earlier concept of oxidation and reduction is based on the addition or removal of oxygen or hydrogen elements so, in terms of oxygen and hydrogen, oxidation is addition of oxygen to a substance and removal of hydrogen from a substance. On the other hand, reduction is addition of hydrogen to a substance and removal of oxygen from a substance. The substance which gives oxygen to another substance or removes hydrogen from another substance in an oxidation reaction is known as oxidising agent, while the substance which gives hydrogen to another substance or removes oxygen from another substance in a reduction reaction is known as reducing agent. For example,
Question.1. A redox reaction is one in which
(a) both the substances are reduced
(b) both the substances are oxidised
(c) an acid is neutralised by the base
(d) one substance is oxidised while the other is reduced.
Question.2. In the reaction, H_{2}S+Cl_{2} \rightarrow S+2HCl
(a) H_{2}S is the reducing agent.
(c) HCl is the oxidising agent.
(b) H_{2}S is the oxidising agent.
(d) Cl_{2} is the reducing agent.
Question.3. Which of the following processes does not involve either oxidation or reduction?
(a) Formation of slaked lime from quick lime.
(b) Heating mercuric oxide.
(c) Formation of manganese chloride from manganese oxide (MnO_{2}).
(d) Formation of zinc from zinc blende.
Question.4. Mg+CuO \rightarrow MgO+Cu
Which of the following is wrong relating to the above reaction?
(a) CuO gets reduced.
(b) Mg gets oxidised.
(c) CuO gets oxidised.
(d) It is a redox reaction.
Question.5. Identify the correct oxidising agent and reducing agent in the following reaction.
Fe_{2}O_{3}+2Al \rightarrow 2Fe+Al_{2}O_{3}
(a) Al – Oxidising agent, Fe_{2}O_{3} – Reducing agent
(b) Fe_{2}O_{3} – Oxidising agent, Al – Reducing agent
(c) Fe – Oxidising agent, Al_{2}O_{3} – Reducing agent
(d) Fe_{2}O_{3} – Oxidising agent, Al_{2}O_{3} – Reducing agent
Ans.1. (d)
In a redox reaction, one reactant is reduced while other reactant is oxidised.
Ans.2. (a)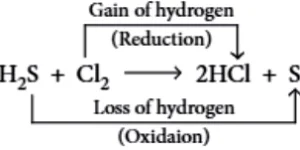
H_{2}S :- Reducing agent
Ans.3. (a)
Formation of slaked lime from quick lime: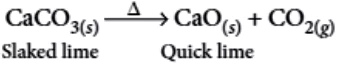
Ans.4. (c)
Addition of oxygen is called oxidation while removal of oxygen is called reduction.
Thus, Mg gets oxidised and CuO gets reduced and it is a redox reaction.
Ans.5. (b)
Case Study Question 06
Read the following and answer any four questions from 1 to 5.
Oxidation has damaging effect on metals as well as on food. The damaging effect of oxidation on metal is studied as corrosion and that on food is studied as rancidity. The phenomenon due to which metals are slowly eaten away by the reaction of air, water and chemicals present in atmosphere, is called corrosion. For example, iron articles are shiny when new, but get coated with a reddish brown powder when left for sometime. This process is known as rusting of iron. Rancidity is the process of slow oxidation of oil and fat (which are volatile in nature) present in the food materials resulting in the change of smell and taste in them.
Question.1. Rancidity can be prevented by
(a) adding antioxidants
(b) packaging oily food in nitrogen gas
(c) both (a) and (b)
(d) none of these
Question.2. Combination of phosphorus and oxygen is an example of
(a) oxidation
(b) reduction
(c) rancidity
(d) none of these
Question.3. A science teacher wrote the following statements about rancidity:
(I) When fats and oils are reduced, they become rancid.
(II) In chips packet, rancidity is prevented by oxygen.
(III) Rancidity is prevented by adding antioxidants.
Select the correct option.
(a) (I) only
(b) (II) and (III) only
(c) (III) only
(d) (I), (II) and (III)
Question.4. Two statements are given below regarding rusting of iron.
(I) The rusting of iron is a redox reaction and reaction occurs as, 4Fe+3O_{2} \rightarrow 4Fe^{3+}+6O^{2-}
(II) The metallic iron is oxidised to Fe^{2+} and O_{2} is reduced to O^{2-} .
Select the correct statement(s).
(a) I only
(b) II only
(c) Both I and II
(d) None of these
Question.5. Which of the following measures can be adopted to prevent or slow down rancidity?
(I) Food materials should be packed in air tight container.
(II) Food should be refrigerated.
(III) Food materials and cooked food should be kept away from direct sunlight.
(a) Only II and III
(b) Only I and III
(c) Only II and III
(d) I, II and III
Ans.1. (c)
Antioxidants and nitrogen gas prevent oxidation of food.
Ans.2. (a)
4P+3O_{2} \rightarrow 2P_{2}O_{3},
4P+5O_{2} \rightarrow 2P_{2}O_{5}
Ans.3. (c)
The oils and fats are slowly oxidised to certain bad smelling compounds, which release foul smell. This is known as rancidity.
Rancidity is prevented by filling nitrogen gas in chips packets.
Ans.4. (a)
Ans.5. (d)
Case Study Question 07
Read the following and answer any four questions from 1 to 5.
Chemical equation is a method of representing a chemical reaction with the help of symbols and formulae of the substances involved in it. In a chemical equation, the substances which combine or react are called reactants and new substances produced are called products. A chemical equation is a short hand method of representing a chemical reaction. A balanced chemical equation has equal number of atoms of different elements in the reactants and products side. An unbalanced chemical equation has unequal number of atoms of one or more elements in reactants and products. Formulae of elements and compounds are not changed to balance an equation.
Question.1. Consider the following reaction:
pMg_{3}N_{2}+qH_{2}O\rightarrow rMg(OH)_{2}+sNH_{3}
When the equation is balanced, the coefficients p, q, r, s respectively are
(a) 1, 3, 3, 2
(c) 1, 2, 3, 2
(b) 1, 6, 3, 2
(d) 2, 3, 6, 2
Question.2. Which of the following information is not conveyed by a balanced chemical equation?
(a) Physical states of reactants and products
(b) Symbols and formulae of all the substances involved in a particular reaction
(c) Number of atoms/molecules of the reactants and products formed
(d) Whether a particular reaction is actually feasible or not
Question.3. The balancing of chemical equations is in accordance with
(a) law of combining volumes
(b) law of constant proportions
(c) law of conservation of mass
(d) both (b) and (c).
Question.4. Which of the following chemical equations is an unbalanced one?
(a) 2NaHCO_{3} \rightarrow Na_{2}CO_{3}+H_{2}O+CO_{2}
(b) 2C_{4}H_{10}+12O_{2} \rightarrow 8CO_{2}+10H_{2}O
(c) 2Al+6H_{2}O \rightarrow 2Al(OH)_{3}+3H_{2}
(d) 4NH_{3}+5O_{2} \rightarrow 4NO+6H_{2}O
Question.5. Which of the following statements is/are correct?
(a) A chemical equation tells us about the substances involved in a reaction.
(b) A chemical equation informs us about the symbols and formulae of the substances involved in a reaction.
(c) A chemical equation tells us about the atoms or molecules of the reactants and products involved in a reaction.
(d) All the above.
Ans.1. (b)
Mg_{3}N_{2}+6H_{2}O \rightarrow 3Mg(OH)_{2}+2NH_{3}
Ans.2. (d)
Ans.3. (c)
In a balanced chemical equation, total mass of reactants must be equal to the total mass of products. This is the statement of law of conservation of mass.
Ans.4. (b)
Ans.5. (d)
Case Study Question 08
Read the following and answer any four questions from 1 to 5.
In decomposition reactions, a single reactant breaks down to form two or more products. Decomposition reaction is opposite to combination reaction. Thermal decomposition reactions use the energy in form of heat for decomposition of reactants. Electrolytic decomposition reactions involve the use of electrical energy for the decomposition of reactant molecules. Photolysis or photochemical decomposition involves the use of light energy for the purpose of decomposition.
Question.1. Which of the following reactions is a decomposition reaction?
(a) NaOH+HCl \rightarrow NaCl+H_{2}O
(b) NH_{4}CNO \rightarrow H_{2}NCONH_{2}
(c) 2KClO_{3} \rightarrow 2KCl+3O_{2}
(d) H_{2}+I_{2} \rightarrow 2HI
Question.2. 2Pb(NO_{3})_{2} \rightarrow 2PbO+nA+O_{2} What is nA in the given reaction?
(a) 4NO
(b) 4NO_{2}
(c) 2PbNO_{2}
(d) NO_{2}
Question.3. Amino acid is formed by the decomposition of which component of our diet?
(a) Carbohydrate
(b) Starch
(c) Protein
(d) Fat
Question.4. Silver chloride on exposure to sunlight for a long duration turns grey due to
(I) the formation of silver by decomposition of silver chloride
(II) sublimation of silver chloride
(III) decomposition of chlorine gas from silver chloride
(IV) oxidation of silver chloride
The correct statement(s) is/are
(a) Only (I)
(b) Only (II) and (III)
(c) Only (I) and (II)
(d) Only (IV)
Question.5. What type of chemical reaction takes place when electricity is passed through water?
(a) Thermal decomposition
(b) Electrolytic decomposition
(c) Photochemical decomposition
(d) Displacement reaction
Ans.1. (c)
Ans.2. (b)
2Pb(NO_{3})_{2} \rightarrow 2PbO+4NO_{2}+O_{2}
Ans.3. (c)
Proteins in our diet get broken down into amino acids.
Ans.4. (a)
2AgCl_{(s)}+Sunlight \rightarrow 2Ag_{(s)}+Cl_{2(g)}
Ans.5. (b)
Electrolysis of water is electrolytic decomposition.
2H_{2}O+Current \rightarrow 2H_{2}+O_{2}
Case Study Question 09
Read the following and answer any four questions from 1 to 5.
Redox reactions are those reactions in which oxidation and reduction occur simultaneously. A redox reaction is made up of two half reactions. In the first half reaction, oxidation takes place and in second half reaction, reduction occurs. Oxidation is a process in which a substance loses electrons and in reduction, a substance gains electrons. The substance which gains electrons is reduced and acts as an oxidising agent. On the other hand, a substance which loses electrons is oxidised and acts as a reducing agent.
Question.1. Which of the following is a redox reaction?
(a) CaCO_{3} \rightarrow CaO+CO_{2}
(b) H_{2}+Cl_{2} \rightarrow 2HCl
(c) CaO+2HCl \rightarrow CaCl_{2}+H_{2}O
(d) NaOH+HCl \rightarrow NaCl+H_{2}O
Question.2. Identify the reaction in which H_{2}O_{2} , is acting as a reducing agent.
(a) H_{2}SO_{3}+H_{2}O_{2} \rightarrow H_{2}SO_{4}+H_{2}O
(b) 2HI+H_{2}O_{2} \rightarrow 2H_{2}O+I_{2}
(c) Cl_{2}+H_{2}O_{2} \rightarrow 2HCl+O_{2}
(d) FeCl_{2}+2HCl+H_{2}O_{2} \rightarrow 2FeCl_{3}+2H_{2}O
Question.3. For the following reactions, identify the one in which H_{2}S acts as a reducing agent.
(a) CuSO_{4}+H_{2}S \rightarrow CuS+H_{2}SO_{4}
(b) Cd(NO_{3})_{2}+H_{2}S \rightarrow CdS+2HNO_{3}
(c) 2FeCl_{3}+H_{2}S \rightarrow 2FeCl_{2}+2HCl+S
(d) None of these
Question.4. For the following reaction, identify the correct statement.
ZnO+CO \rightarrow Zn+CO_{2}
(a) ZnO is being reduced.
(b) CO_{2} is being reduced.
(c) CO is being oxidised.
(d) ZnO is being oxidised.
Question.5. In the following reaction, which substance is reduced?
PbS+4H_{2}O_{2} \rightarrow PbSO_{4}+4H_{2}O
(a) H_{2}O
(b) H_{2}O_{2}
(c) PbS
(d) PbSO_{4}
Ans.1. (b)
H_{2} is oxidised to HCl while Cl_{2} is reduced to HCl.
Ans.2. (c)
Ans.3. (c)
2FeCl_{3}+H_{2}S \rightarrow 2FeCl_{2}+2HCl+S
H_{2}S itself gets oxidised to S and reduces FeCl_{3} to FeCl_{2}.
Ans.4. (a)
ZnO is reduced to Zn and CO is oxidised to CO_{2}.
Ans.5. (b)
H_{2}O_{2} is reduced to water by removal of oxygen.
Case Study Question 10
Read the following and answer any four questions from 1 to 5.
In a balanced chemical reaction, equal number of atoms are present on both sides of reaction. A balanced chemical reaction is based on law of conservation of mass which means that total mass of reactants and products participating in a reaction must be equal. For example, a balanced chemical equation of burning of magnesium in oxygen to form magnesium oxide is written as :
2Mg+O_{2} \rightarrow 2MgO
The mass of reactants (2\times 24+32=80) is equal to the mass of products [2\times (24+16)=80].
Question.1. In a reaction, 35 g of reactant, PQ breaks down into 20 g of product, Pand an unknown amount of product, Q. Using the law of conservation of mass, weight of products, Q will be
(a) 25 g
(b) 35 g
(c) 30 g
(d) 15 g
Question.2. When solid mercury (II) oxide is heated, liquid mercury and oxygen gas are produced. Which of the following statements is true regarding the balanced chemical equation for this process?
(a) 1 mole of mercury (II) oxide produces two moles of mercury and one mole of oxygen gas.
(b) 2 moles of mercury (II) oxide produce one mole of mercury and one mole of oxygen gas.
(c) 1 mole of mercury (II) oxide produces half mole of mercury and half mole of oxygen gas.
(d) 2 moles of mercury (II) oxide produce 2 moles of mercury and one mole of oxygen gas.
Question.3. Which of the following laws is satisfied by a balanced chemical equation?
(a) Law of multiple proportions
(b) Law of conservation of mass
(c) Law of conservation of motion
(d) Law of conservation of magnetism
Question.4. In the given chemical reaction,
2C_{6}H_{6(l)}+15O_{2(g)} \rightarrow mCO_{2(g)}+nH_{2}O_{(l)}
The values of m and n are respectively
(a) 14 and 8
(b) 12 and 6
(c) 8 and 10
(d) 12 and 10
Question.5. Sulphur dioxide reacts with oxygen to form sulphur trioxide. What would be the molar ratio of sulphur dioxide to sulphur trioxide?
(a) 2:3
(b) 1:1
(c) 1:2
(d) 3:2
Ans.1. (d)
PQ{(35g)} \rightarrow P {(20g)}+Q{(?)}
According to law of conservation of mass,
Mass of PQ = Mass of P + Mass of Q
\therefore Mass of Q = (35-20)g = 15 g
Ans.2. (d)
2HgO_{(s)} \rightarrow 2Hg_{(l)}+O_{2(g)}
Ans.3. (b)
Ans.4. (b)
Ans.5. (b)