Competency Based Questions for Class 10 Science Chapter 4 Carbon and Its Compounds
Competency Based Questions are new type of questions asked in CBSE Board exam for class 10. Practising the following Competency Based Questions will help the students in facing Board Questions.
Hint: Write down electron shell configuration of carbon in order to predict formulae of carbon compounds and illustrate the structure of molecules of carbon compounds with chain, branched & ring structure.
Question.1. What is the electronic configuration of carbon?
(a) 2, 4
(b) 2, 8
(c) 2, 2, 4
(d) 2, 4, 4
Answer. (a) 2, 4
Question.2. The electronic configuration of an element is found to be 2, 4. How many bonds can one carbon atom form in a compound?
(a) 1
(b) 2
(c) 4
(d) 6
Answer. (c) 4
Hint: Draw structures of carbon compounds in order to classify them as saturated or unsaturated.
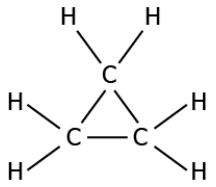
Question.3. Which of these compounds can be classified as an unsaturated compound?
(a) 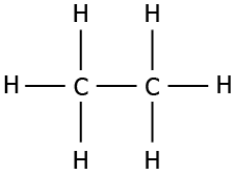
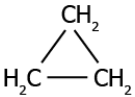
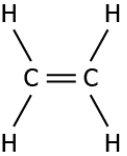
Answer. (c)
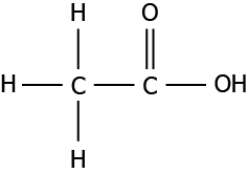
Question.4. A student studies that acetic acid is a saturated compound. The structure of the compound is shown. 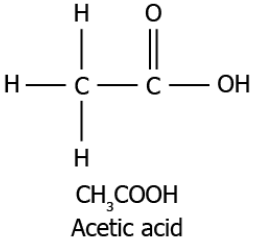
(a) because there is a the single bond between the carbon atoms
(b) because there is a double bond between the carbon and oxygen atoms
(c) because there is a single bond between the carbon and hydrogen atoms
(d) because there is a single bond between the carbon and hydroxide diatom
Answer. (a) because there is a the single bond between the carbon atoms
Hint: Draw structures of carbon compounds and show types of bonds (single/ double/ triple) in order to classify them as alkanes/ alkenes/ alkynes.
Question.5. Which of these carbon compounds represents an alkene
(a) (b)
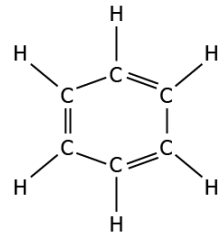
Answer. (b)
Question.6. The image represents the structure of a few hydrocarbon compounds.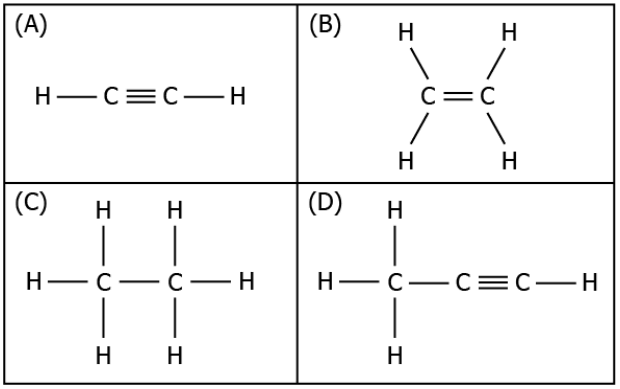
(a) only (A)
(b) only (B)
(c) both (A) and (D)
(d) both (B) and (C)
Answer. (c) both (A) and (D)
Hint: Draw structures of carbon compounds with functional groups, in order to predict their properties due to functional groups and type of bonding present.
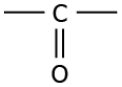
Question.7. The image represents a carbon compound. 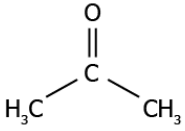
(a) alcohol
(b) aldehyde
(c) carboxylic acid
(d) ketone
Answer. (d) ketone
Question.8. Which of these functional groups can combine with carbon to produce alcohol?
(a) 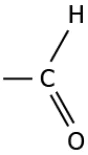
(c)
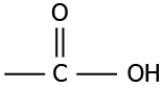
Answer. (b)
Hint: Classify carbon compounds in homologous series in order to predict their properties.
Question.9. Which of these series can be classified as homologous series?
(a) CHCl_{3} , C_{2}H_{5}OH , C_{3}H_{7}OH
(b) CH_{3}OH , C_{2}H_{5}OH , C_{3}H_{7}OH
(c) CHCl_{3} , C_{4}H_{9}OH , CH_{3}COOH
(d) CH_{3}COOH , C_{4}H_{9}OH , C_{2}H_{5}OH ♪
Answer. (b) CH_{3}OH , C_{2}H_{5}OH , C_{3}H_{7}OH
Question.10. A student studies that the carbon compounds CH_{3}OH , C_{2}H_{5}OH , C_{3}H_{7}OH and C_{4}H_{9}OH can be group as homologues series. Why are these compounds grouped as homologous series?
(a) because of an increase in number of carbon atom along the series
(b) because of an increase in number of hydrogen atom along with the series
(c) because of the presence of the same functional group substitute for hydrogen in a carbon chain
(d) because of the presence of the same carbon compounds substitute for hydrogen in a carbon chain
Answer. (c) because of the presence of the same functional group substitute for hydrogen in a carbon chain
Hint: Identify the functional group, type of bonding, number of C atoms present in a carbon compound, in order to correctly name them.
Question.11. A carbon compound contains two atoms of carbon. Which name should the carbon compound bear?
(a) Butane
(b) Ethane
(c) Methane
(d) Propane
Answer. (b) Ethane
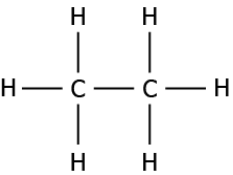
Question.12. The image represents the structure of a carbon compound known as ethane. 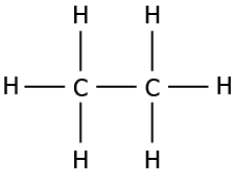
(a) the presence of functional group connected with a single bond
(b) as it contains two carbon atoms and a single bond connects the carbon atoms
(c) carbon compound with a total number of eight atoms are named as ethane
(d) as it contains six hydrogen atoms and a single bond connects the carbon and hydrogen atom
Answer. (b) as it contains two carbon atoms and a single bond connects the carbon atoms
Hint: Observe how carbon compounds burn in oxygen, in order to classify them as saturated or unsaturated.
Question.13. A student conducts an activity where he burns methane in the presence of oxygen. What is likely to form?
(a) Water
(b) carbon dioxide
(c) carbon dioxide and water
(d) carbon dioxide and oxygen
Answer. (c) carbon dioxide and water
Question.14. A student conducts an activity, where he took a naphthalene ball and burn it. He observed that it gives a yellow flame with lots of black smoke and sooty deposits around it. What type of hydrocarbon does naphthalene contain?
(a) unsaturated, as black smoke represents complete combustion
(b) unsaturated, as sooty deposit represents unburnt hydrocarbons
(c) saturated, as it gives a yellow flame which represents complete combustion
(d) saturated, as the burning of any substance represents a complete combustion
Answer. (b) unsaturated, as sooty deposit represents unburnt hydrocarbons
Hint: Illustrate the chemical properties of carbon compounds (like combustion, oxidation, addition & substitution) along with balanced chemical reaction.
Question.15. The reaction represents the conversion of alcohol into acids. 
(a) Heat
(b) CH_{3}COOH
(c) CH_{3}CH_{2}OH
(d) Alkaline KMnO_{4}
Answer. (d) Alkaline KMnO_{4}
Question.16. The image represents a chemical reaction where ethanol is oxidised using potassium dichromate and sulphuric acid. 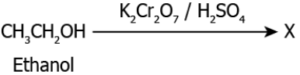
(a) CH_{2}O
(b) CH_{3}CH
(c) CH_{3}H_{2}O
(d) CH_{3}COOH
Answer. (d) CH_{3}COOH
Hint: Identify how carbon compounds react with hydrogen in the presence of nickel catalyst, in order to write a balanced chemical reaction.
Question.17. The image represents the chemical reaction of an unsaturated hydrocarbon in the presence of nickel. 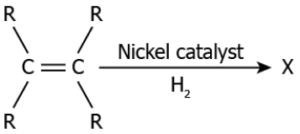
(a) 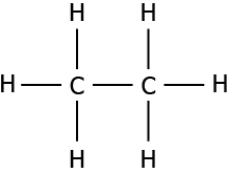
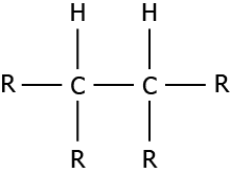
Answer. (c)
Question.18. The image represents a chemical reaction where an unsaturated hydrocarbon is converted into a saturated hydrocarbon in the presence of a catalyst.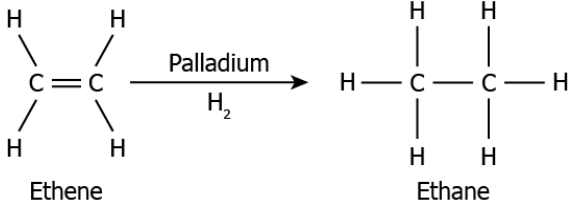
(a) it causes a reaction to proceed without the reaction itself being affected
(b) it causes the removal of all hydrogen atom bonded to the carbon atom
(c) causes to change the single bonds to double and triple bonds
(d) causes the production of oxygen during the reaction
Answer. (a) it causes a reaction to proceed without the reaction itself being affected
Hint: Identify how carbon compounds react with chlorine in the presence of sunlight, in order to write a balanced chemical reaction.
Question.19. The chemical reaction shows the addition of chlorine to methane in the presence of sunlight.
CH_{4}+Cl_{2} \rightarrow X
What is likely to be the product of the reaction represented by “X”?
(a) CH_{4}+H_{2}SO_{4}
(b) CH_{3}Cl+HCl
(c) CHCl_{3}+HCl
(d) CH_{3}Cl+H_{2}SO_{4}
Answer. (b) CH_{3}Cl+HCl
Hint: Identify how carbon compounds react with chlorine in the presence of sunlight, in order to write a balanced chemical reaction.
Question.20. The chemical reaction shows the addition of chlorine gas to hydrocarbon in the presence of sunlight.
CHCl_{3}+Cl_{2} \rightarrow CCl_{4}+HCl
How does chlorine react to a hydrocarbon compound in the presence of sunlight?
(a) it adds hydrogen into the compound
(b) it adds an oxygen atom into the compound
(c) it substitutes hydrogen atom from the compound
(d) it breaks double and triple bonds into a single bond
Answer. (c) it substitutes hydrogen atom from the compound
Hint: Perform physical and chemical tests in order to distinguish between Ethanol & Ethanoic acid based on their properties (reaction with other substances).
Question.21. A student studies that vinegar, which is a diluted form of ethanoic acid, freezes during winter. What does this suggest about the physical properties of pure ethanoic acid?
(a) it has a low boiling point
(b) it has a low melting point
(c) it has a very high boiling point
(d) it has a very high melting point
Answer. (b) it has a low melting point
Question.22. A student conducts an activity where he took ethanoic acid and ethanol in the presence of an acid catalyst. He noticed that the resulted product has some sweet-smelling fragrance. What is likely to be the product?
(a) CH_{3}COOC_{2}H_{5} + H_{2}O
(b) CH_{3}COOH + H_{2}O
(c) C_{2}H_{5}OH + H_{2}O
(d) COOH + H_{2}O
Answer. (a) CH_{3}COOC_{2}H_{5} + H_{2}O
Hint: Describe the process of micelle formation in order to understand how soaps work.
Question.23. A student studies that a soap molecule has two ends, one of which is an ionic end and the other is the carbonic chain. Which option explains the interaction of a soap molecule with oil?
(a) ionic end of the soap interacts with the oil
(b) the closest end of the soap interacts with the oil
(c) carbonic chain end of the soap interacts with the oil
(d) ends of the soap randomly interact with the oil
Answer. (c) carbonic chain end of the soap interacts with the oil
Question.24. A student studies that soap solution results in micelle formation which helps to remove dirt. It has a unique orientation which helps in keeping the dirt out of the water as shown in the image. 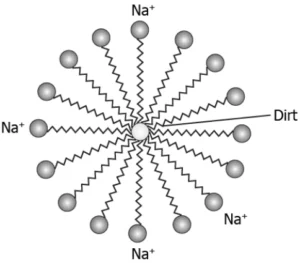
(a) suspension of the dirt in the micelles
(b) a collection of water molecules in the centre of the micelle
(c) attraction between the ionic end and the dirt to remove it
(d) mixing of the soap molecules along with the dirt to make it heavier
Answer. (a) suspension of the dirt in the micelles